Abstract
Background: Venetoclax combined with hypomethylating agents is a new standard of care for newly diagnosed patients with acute myeloid leukemia (AML) 75 years or older, or unfit for intensive chemotherapy. As precision therapy in AML expanded with the addition of venetoclax among others in the therapeutic armamentarium of AML, efficacy and safety reports in ethnic minorities are limited, with a background of well recognized inter-ethnic differences in drug response. Phase III data from VIALE-A, as well as VIALE-C, was limited for the Arab population as no site opened in the Arab world. We herein report our experience on the use of venetoclax with azacitidine in patients with newly diagnosed or relapsed/refractory AML in the Arab population.
Methods: Retrospective-single center review on the use of Azacitidine with venetoclax in older patients (aged ≥60 years) with newly diagnosed AML, not eligible for intensive chemotherapy; secondary AML and relapsed or refractory AML. All patients self-identified of Arabic ethnicity. Patients who received previous BCL2-inhibitor therapy were excluded. Patients who received at least one dose of treatment (Azacitidine ≥3 days, >14 days of venetoclax) were included in the intention to treat analysis. Patients typically received azacitidine 75 mg/m2 intravenously for 7 days with oral venetoclax 400 mg daily for induction, with appropriate dose adjustment for concomitant use of azoles. This is followed by the same regimen in consolidation, with adjustment according to response and side effects at the treating physician's discretion. The primary endpoint was overall survival. The secondary endpoints include response rate, safety, and relapse-free survival.
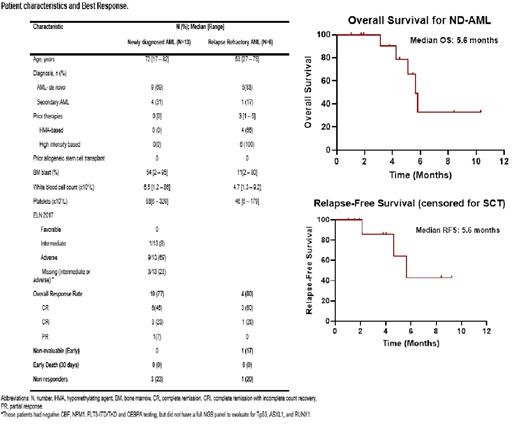
Results: Between July 2019, and July 2021, we identified 19 patients; 13 (68%) had newly diagnosed AML (ND-AML), and 6 (32%) had relapsed or refractory AML (R/R AML). The median age was 70 years (17-82). In the ND-AML, most patients had an adverse ELN 2017 AML (69%) with 23% having either intermediate or adverse AML (Negative for CBF, NPM1, FLT3-ITD and biCEBPA, but missing NGS data for adverse mutations Tp53/ASXL1 and RUNX1). Only one patient was classified as intermediate-risk AML. The overall response rate in the ND-AML was 77%, with 46% achieving complete remission (CR), and 23% CR with incomplete count recovery (CRi) [Table]. One patient achieved PR after the first cycle (blast 7% by morphology and 1.5% by flow cytometry) and did not have a subsequent bone marrow evaluation, however had a full count recovery. Among the responders in the ND-AML cohort, 4 deaths were noted. One death was related to COVID-19 associated pneumonia, one due to graft failure (at day 42 post Haplo-SCT), one due to septic shock, and one was related to relapse disease. The overall survival and relapse-free survival for ND-AML were 5.6 months for both [Figure].
In the R/R AML, 66% had prior HMA exposure, and all patients did receive high-intensity chemotherapy. The median number of prior treatments was 3 (1-5). the response rate was 80% (4/5), with 60% achieving CR. All patients are still alive with a median follow-up of 7.6 months. One patient had progressive disease. One patient is early to evaluate and was not included in the response analysis [Table]. The 30-day mortality was zero in both ND-AML and R/R AML cohorts.
Conclusions: In a majority of adverse risk ND-AML, and in heavily pretreated R/R AML, the response rate and overall survival is comparable to what has been previously reported. Our data support the use of this regimen in older patients with newly diagnosed AML, patients with relapsed or refractory disease, and those with adverse-risk features. This analysis is limited by the small number of patients, and by the lack of ELN 2017 favorable-risk AML. Future prospective and randomized studies are needed to clarify activity and safety in the Arab population, as well as in the high-risk AML subset.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal